Water
Essential to all life, water is the foundation upon which life is built. Water is a common solvent, or dissolving medium, for countless chemical reactions. The chemical reactions that take place in water are called aqueous solutions. To understand the nature of these aqueous solutions, we must first understand the nature of water itself.One of water's most important abilities is its ability to dissolve many substances. This dissolving can only be understood by first learning the molecular structure of the water molecule: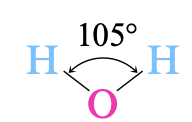
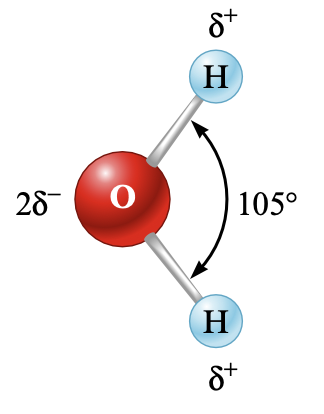

Differing ionic substances have a different amount of solubility. While sodium chloride is very soluble in water, silver chloride is only slightly soluble. The amount of solubility is highly dependent on the force of attraction between the water and the anions in the ionic solid.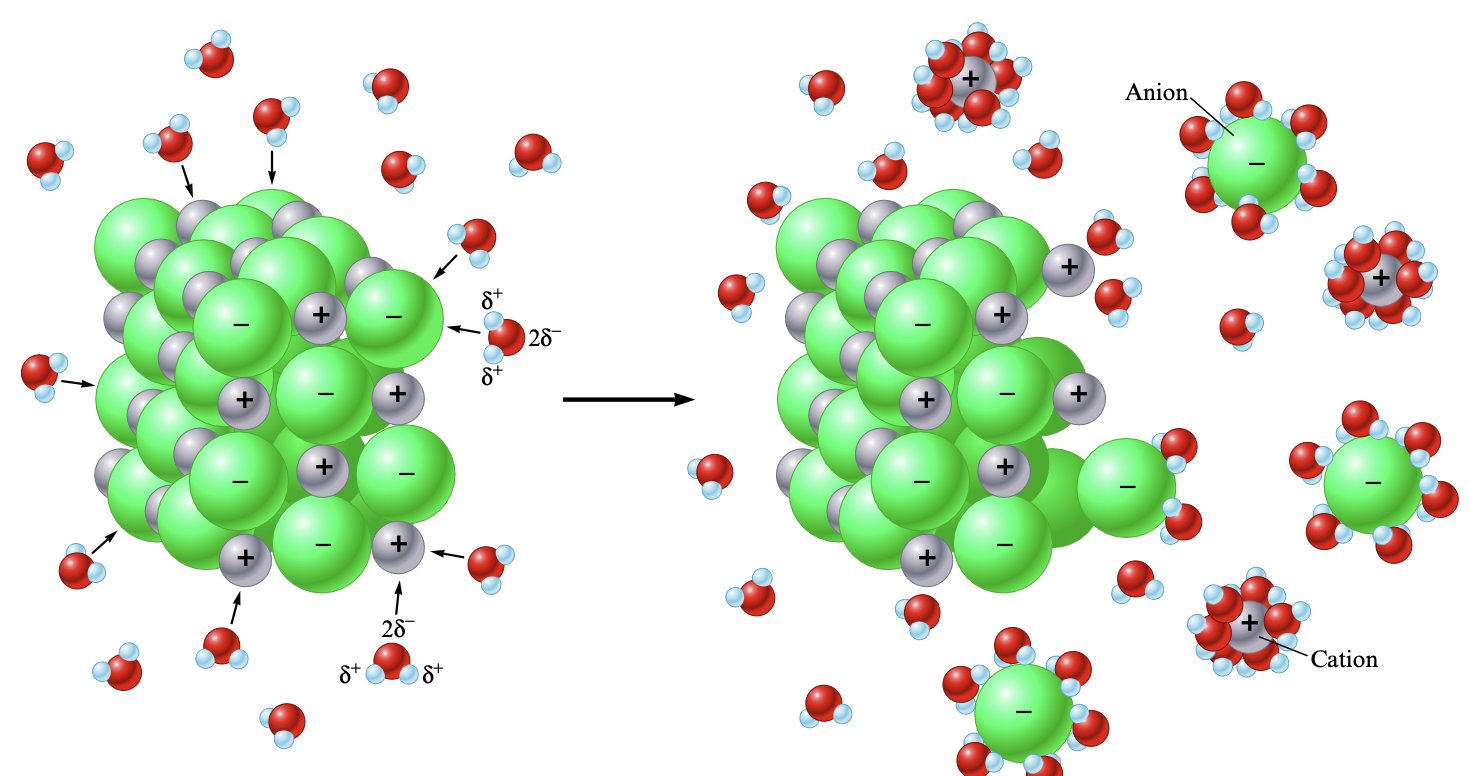
Strong and Weak Electrolytes
The substance being dissolved in a solution is called the solute and the solution itself is called the solvent. A solution can be characterized by its electrical conductivity, or its ability to conduct an electrical current. If a solution can conduct electricity effectively, it usually contains strong electrolytes. If it doesn't, the solution contains weak electrolytes. If the solution does not conduct any electricity at all, the solution contains nonelectrolytes.Strong electrolytes are completely ionized when dissolved in water. They are usually either soluble salts, strong acids, or strong bases. For example, NaCl (sodium chloride) forms ions when dissolved in water. This makes it a soluble salt and a strong electrolyte. The concept of acids is strongly related to electrolytes. An acid is a substance that produces H+ ions, or protons, when dissolved in water. When an acid of the form HA is dissolved in water, the following ionization reaction occurs: 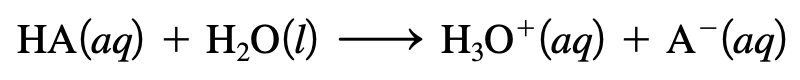
While strong electrolytes can be strong acids, they can also be strong bases. Strong bases are soluble ionic compounds that contain the hydroxide ion (OH-). Solutions with bases usually have a bitter taste and slippery feel, similar to soap (which is a base).
Weak electrolytes are weak acids and bases that do not create many ions when dissolved in water. Nonelectrolytes do dissolve in water, but they do not produce any ions at all.
Solution Calculations
The molarity of a solution is the number of moles of solute per volume of the solution in liters.$$M = \text{molarity} = \frac{\text{moles of solute}}{\text{liters of solution}}$$ A solution with a molarity of 1 M contains 1 mole of solute per liter of solution.When doing experiments, scientists often need a solution with a certain molarity. Unfortunately, such solutions may not be available for purchase, and instead a higher or lower molarity solution is being sold on the market. To get the desired solution, chemists must dilute the stock solution. This can be done by adding pure solvent to the solution until the final molarity of the solution has been achieved. Dilution usually requires a pipet and volumetric flask to accurately measure and transfer solution from one container to another.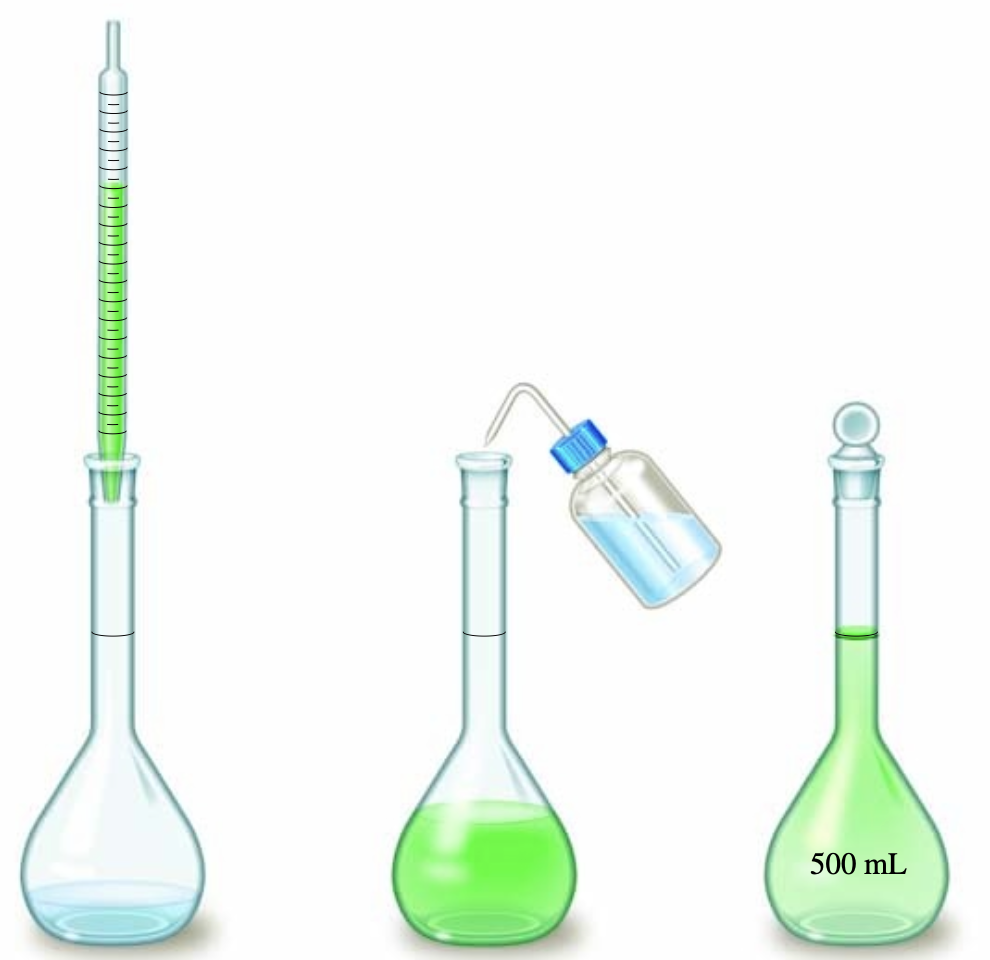
Types of Chemical Reactions
Chemical reactions can be grouped into 3 classes: precipitation reactions, acid-base reactions, and oxidation-reduction reactions. Here's a bullet point list for each of the three types:- Precipitation Reactions
- Precipitation reactions are when a precipitate (solid) is formed and separated from a liquid solution.
- To determine the nature of the precipitate, the identities of the reactants and other products must be known. Then, balance the chemical equation based on the already known possible solid compounds.
- The compounds must have a 0 net charge.
- The ionic materials must contain one type of cation and one type of anion.
- Use the known color of different compounds to identify the solid.

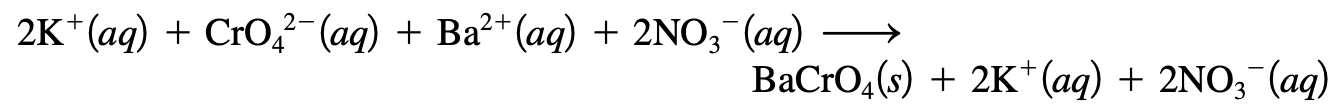
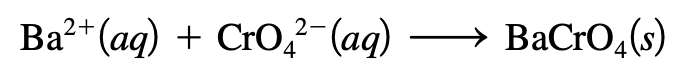
- The formula equation gives the overall reaction stoichiometry but not the actual ions.
- The complete ionic equation shows the reactants as ions and displays the products that are strong electrolytes.
- The net ionic equation only shows the reaction components that have changed from the left side of the reaction to the right.
Acid-Base Reactions
An acid is a proton donor and a base is a proton acceptor.In acid-base reactions, the hydrogen ion from the acid will combine with the hydroxide from the base to create water. This concept can be seen in the following acid-base reaction between potassium hydroxide (KOH) and acetic acid (HC2H3O2). $$\text{KOH} + \text{HC}_2\text{H}_3\text{O}_2 \rightarrow \_\_\_\_\_\_$$ Separate the ions:$$\text{K}^+ + \text{OH}^- + \text{H}^+ + \text{C}_2\text{H}_3\text{O}_2^- \rightarrow \_\_\_\_\_\_$$Now we can see that the hydrogen and hydroxide ions will combine to form water:$$\text{OH}^- + \text{HC}_2\text{H}_3\text{O}_2 \rightarrow \text{H}_2\text{O} + \text{C}_2\text{H}_3\text{O}_2^-$$Usually, in chemistry, we consider the hydroxide ion to be such a strong base that it reacts completely with any weak acid that it encounters. When enough base is added to an acid, the acid is said to be neutralized and a neutralization reaction has taken place.
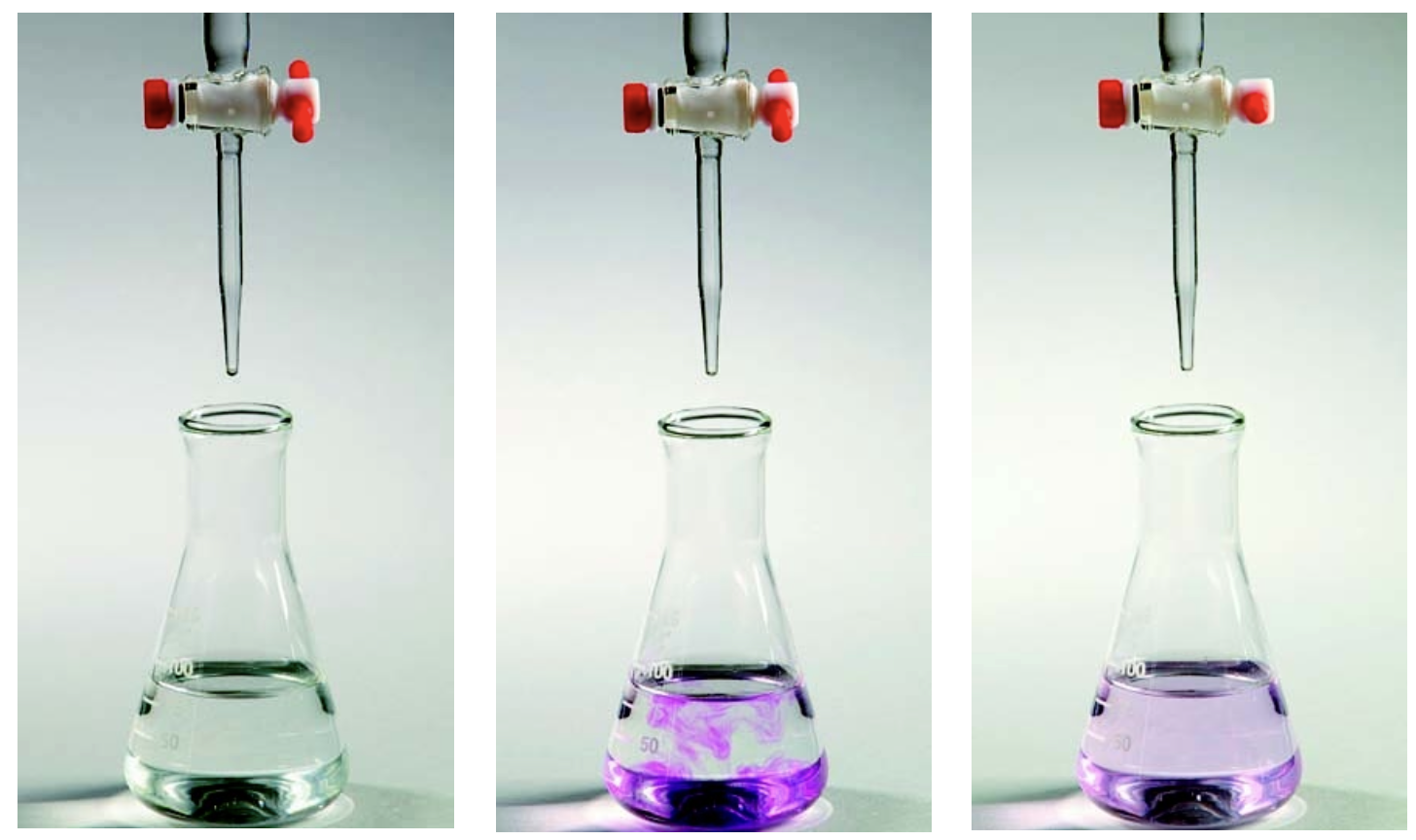
Acid-Base Titrations
Titrations are used to identify the exact point at which enough reactant has been added to completely finish the entire reaction. When enough reactant has been added to react with the analyte (substance be analyzed), the equivalence point, or stoichiometric point, has been reached. We can identify when this point has been reached by observing an indicator which usually changes color near the equivalence point. When the indicator actually changes color, the endpoint of the titration has been reached.These are the requirements to do a successful titration:
- The reaction between the titrant and analyte must be rapid and known.
- Stoichiometric or equivalence point must be marked accurately.
- Volume of titrant added must be accurately measured at each point.
Oxidation-Reduction Reactions
Oxidation-reduction reactions, or redox reactions, are reactions in which one or more electrons are transferred. Photosynthesis in plants is an important redox reaction in nature. While the reactants and product may not be ionic, redox reactions can still occur. To understand oxidation reduction reactions, we must first understand oxidation states. The oxidation state of an atom is the imaginary charge the atom would have if the shared electrons were divided equally between identical atoms or given to the atom with a higher electronegativity if the atoms are different. The sum of the oxidation states must be 0 for an electrically neutral compound and for an ion, the sum of the oxidation states must be equal to the ion's charge. The table below makes asigning oxidation states much simpler: