Fundamental Chemical Laws
The first fundamental chemical law was created by the French chemist Antoine Lavoisier, who claimed that mass can neither be created nor destroyed, a rule that is now known as the law of conservation of mass.In the 1800s, the French scientist Joseph Proust created Proust's law, or the law of definite proportions. This law states that any given compound always contains exactly the same proportion of elements by mass. For example, water is always 88.8% oxygen and 11.2% hydrogen by mass regardless of how much water you have.
After learning of Proust's discovery, John Dalton, an English schoolteacher began to think of each element as being composed of its own atom. This would explain why the proportions of elements in a compound are always the same. Dalton also noticed that the same elements can combine in different numbers to form different compounds. Carbon and oxygen can combine to form either carbon monoxide (CO) or carbon dioxide (CO2), two very different gases. Since this principle applied to other compounds as well, Dalton created the law of multiple proportions, which states that when two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the first element can always be reduced to small whole numbers. In other words, you cannot have a fraction of an atom in any compound.
Dalton's Atomic Theory
Dalton provided his atomic theory in his book A New System of Chemical Philosophy. The theory follows:- Each element is composed of atoms.
- Atoms of the same element are identical and atoms of different elements are different.
- Chemical compounds are made up of atoms of different elements. A compound always has the same relative number and types of atoms. (Only thing that will change is the 'multiplier,' e.g. CO2 vs C2O4).
- Chemical reactions reorganize atoms and change the ways in which they are bound together. The atoms in a chemical reaction are never changed.

And then came Amadeo Avogadro. Using experimental data by Gay-Lussac, Avogadro created Avogadro's hypothesis, which stated that at the same temperature and pressure, equal volumes of different gases contain the same number of particles. This hypothesis was later proven correct and is now known as Avogadro's law, a fundamental rule of gases.
The Early Atom
Now that scientists had discovered the atom, they began to understand more about the individual parts of it. The first major advancement in the understanding of the atom was the discovery of electrons by English physicist J.J. Thomson. While studying electrical discharges in cathode ray tubes, Thomson found that a 'ray' was produced. This ray began from the negative electrode and ended at the positive electrode. When an electric field was applied to this ray, it would curve away from the negative pole of the field, as shown in the diagram below.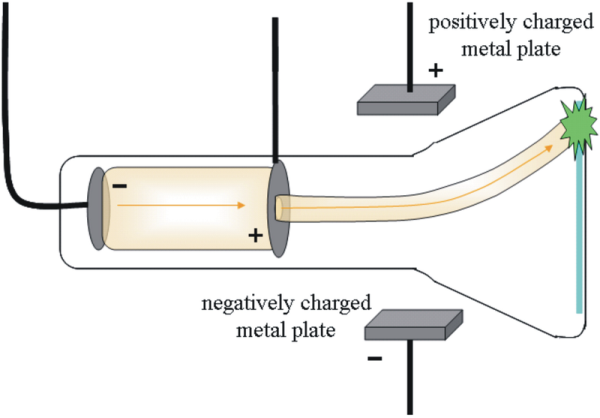
Thomson reasoned that since electrons could be produced from different types of metals, all atoms must contain electrons. Additionally, since atoms are electrically neutral, the atoms must also contain a positive charge to balance the atom out. Thomson came up with his own model of the atom, one that is called the plum pudding model because the raisins (electrons) are placed on the surface of a pudding (positively charged cloud) like the favorite English dessert of the same name.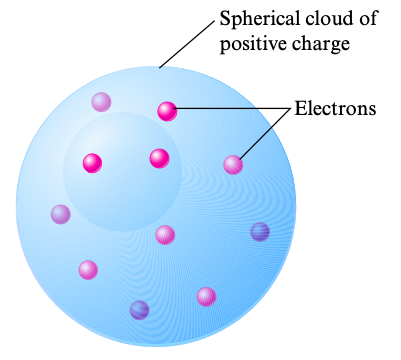
In 1896, the French scientist Henry Becquerel accidentally discovered that a mineral containing uranium produced an image on a photographic plate without having any light shining on it. He believed that this was caused by radioactivity. Later, scientists established 3 forms of radioactivity: gamma rays, beta particles, and alpha particles. Gamma rays are high energy light waves, beta particles are high speed electrons, and alpha particles have a positive charge equal to double the absolute value of an electron's charge.
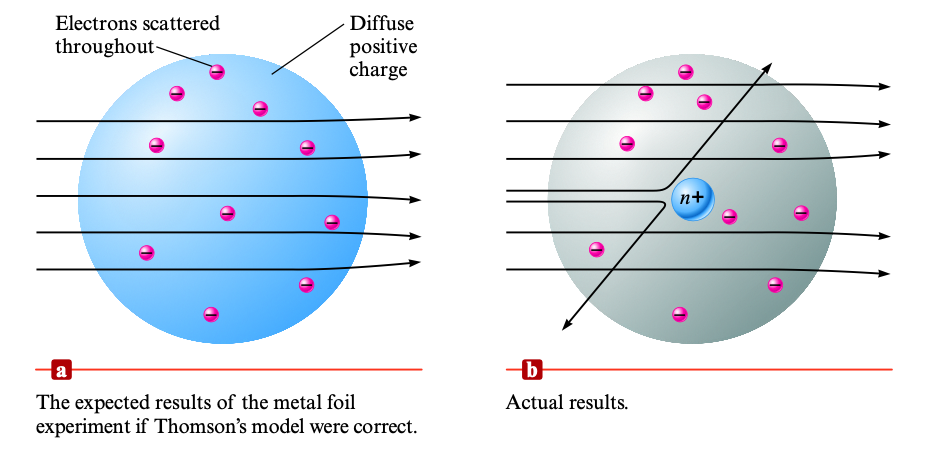
Finally, Ernest Rutherford entered the scene. Unsure about Thomson's plum-pudding model, Rutherford decided to set up an experiment to test Thomson's model. He directed a stream of alpha (\(\alpha\)) particles toward a thin sheet of metal foil. If Thomson's model was correct, the alpha particles would mostly travel through the cloud and straight onto the foil on the other end. Surprisingly, Rutherford found out that some of the particles were deflected at very large angles, angles that couldn't have occurred with only electrons.